With the new European HTA regulation for medical devices and diagnostics, one of the big questions is what impact these evaluations would have on market access for Medtech products.
Except for Belgium and France, other European countries do not have a link between HTA evaluation and reimbursement. In other countries, the HTA evaluations have a different degree of influence on the market access process, from very loose to a very direct impact.
The figure below provides an overview of how often different countries have cited the EUnetHTA reports.
With our understanding of these markets, we would suggest a few perspectives that may be helpful to understand the potential impact of the EU-HTA regulation for Medtech:
- Countries with small HTA organizations are more interested in leveraging the combined work.
- Austria has a straightforward utility of HTA in the decision-making of new Medtech products nationally.
- Italy and Spain broadly use HTA on a regional and increasingly on a national level.
- France had one reference and Germany none. We would suggest this is primarily due to their capacity and well-defined scope of when to do HTA.
Interested to learn more about this? Don’t miss our upcoming webinar on May 8th:
If you want to learn more about how we can support you with the EU HTA regulation, please contact us.
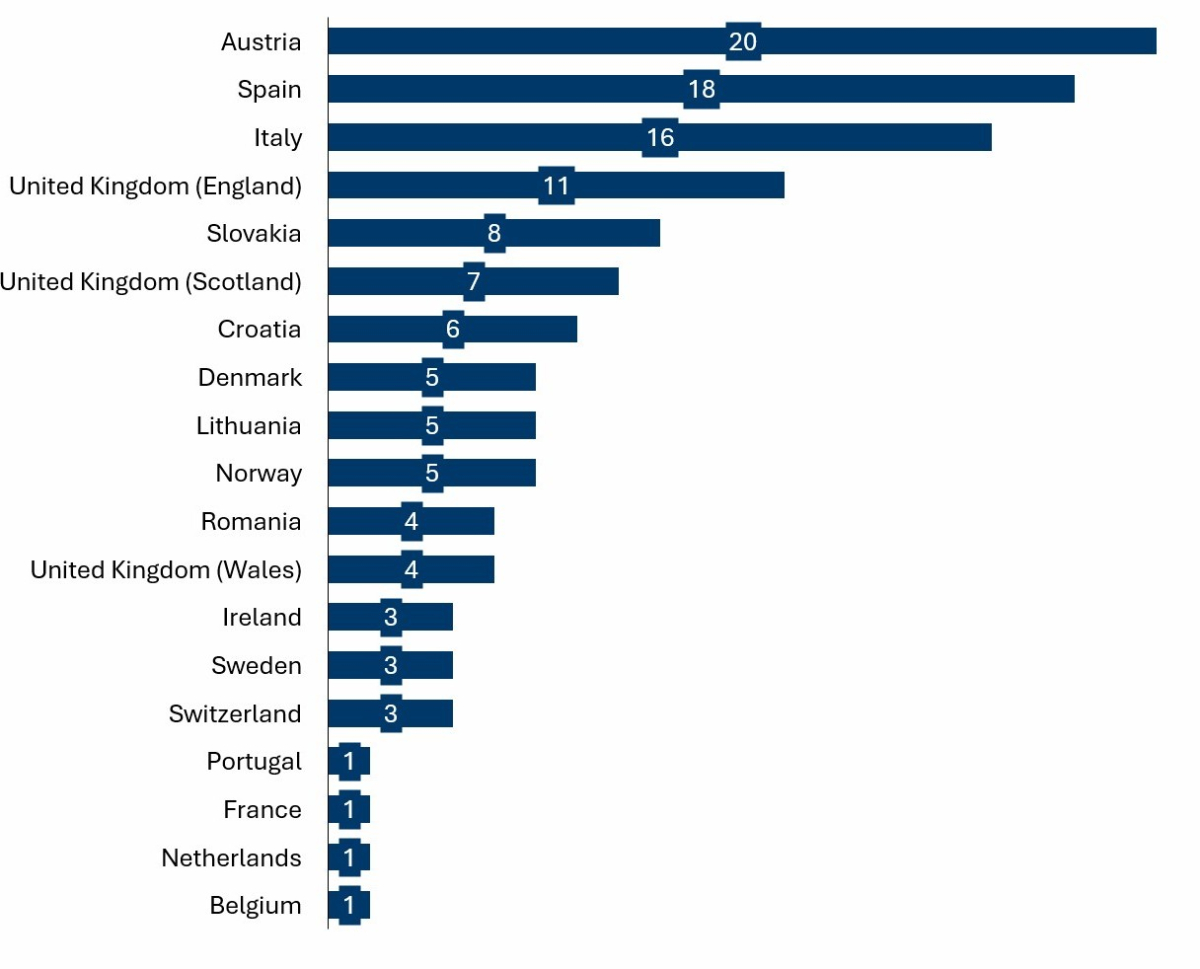
Figure 1 - Frequency of referencing EUnetHTA report
These are the Medtech projects from EUnetHTA:
- OTCA01 Wearable cardioverter-defibrillator (WCD) therapy in primary and secondary prevention of sudden cardiac arrest in patients at risk
- OTCA02 Antibacterial-coated sutures versus non-antibacterial-coated sutures for the prevention of abdominal, superficial and deep, surgical site infection (SSI)
- OTCA05 Repetitive transcranial magnetic stimulation for treatment-resistant major depression
- OTCA06 Transcatheter aortic valve implantation (TAVI) in patients at intermediate surgical risk
- OTCA07 Relative effectiveness assessment of Femtosecond laser-assisted cataract surgery (FLACS) compared to standard ultrasound phacoemulsification cataract surgery
- OTCA09 High-intensity focused ultrasound (HIFU) ablation for the treatment of prostate cancer
- OTCA11 Custom-made or customisable 3D printed implants and cutting guides versus non-3D printed standard implants and cutting guides for improving outcome in patients undergoing knee, maxillofacial, or cranial surgery
- OTCA14 Robot-assisted surgery in thoracic and visceral indications
- OTCA15 Irreversible electroporation in liver and pancreatic cancer
- OTCA16 Bioresorbable Stents in cardiovascular indications (coronary artery disease)
- OTCA18 Regional hyperthermia for high-risk soft tissue sarcoma treatment
- OTCA20 Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)
- OTCA21 Hypoglossal nerve stimulation systems for treatment of obstructive sleep apnoea
- OTCA23 Biodegradable rectum spacers to reduce toxicity for prostate cancer
- OTCA24 A pulse wave analysis device (Mobil-O-Graph) and ARC solver algorithm to identify cardiovascular frailty
- OTCA25 Stereotactic Body Radiation Therapy (SBRT) for lung, prostate and hepatic cancer
- OTCA26 Comparative effectiveness of surgical methods for treating people with morbid obesity
- OTCA27 Comparative Effectiveness of surgical techniques and devices for the treatment of benign prostatic hyperplasia (BPH)
- OTJA08 Continuous glucose monitoring (CGM real-time) and flash glucose monitoring (FGM) as personal, standalone systems in patients with diabetes mellitus treated with insulin

