
Traditional way to demonstrate value of technology to customers is through different types of marketing materials (brochures, papers etc.). However, typically limited in size marketing materials fail to fully present value or technology in a methodologically sound and compelling way.
There are number of value demonstration formats which are used by industry:
- Core value dossier
- Comprehensive overview of the value proposition for medical technology
- Used to form reimbursement/HTA submission documents (externally), communicate about technology to external stakeholders and train personnel on the value of the technology internally
- Usually includes of 60-150 pages
- “Light” value dossier
- Simplified / short version of core value dossier
- Usually includes 15-30 pages
- Value summary
- MS PowerPoint analogue of “light” value dossier
- Usually includes 15-20 slides
- Business case
- Document, developed for specific stakeholder or type of stakeholders, aimed on demonstrating impact of introduction of a technology on organization
- It is much more pragmatic and practical document, compared with value dossier
Value dossier typically covers number of topics:
- Epidemiology and burden of disease
- Current standard of care and its limitations
- Overview of technology in scope
- Systematic literature review for technology
- Health economic analysis for technology
- Recommendations in clinical guidelines and health technology assessments
- Ethical considerations
- Integration of novel technology into existing patient’s pathway
- Current reimbursement for technology
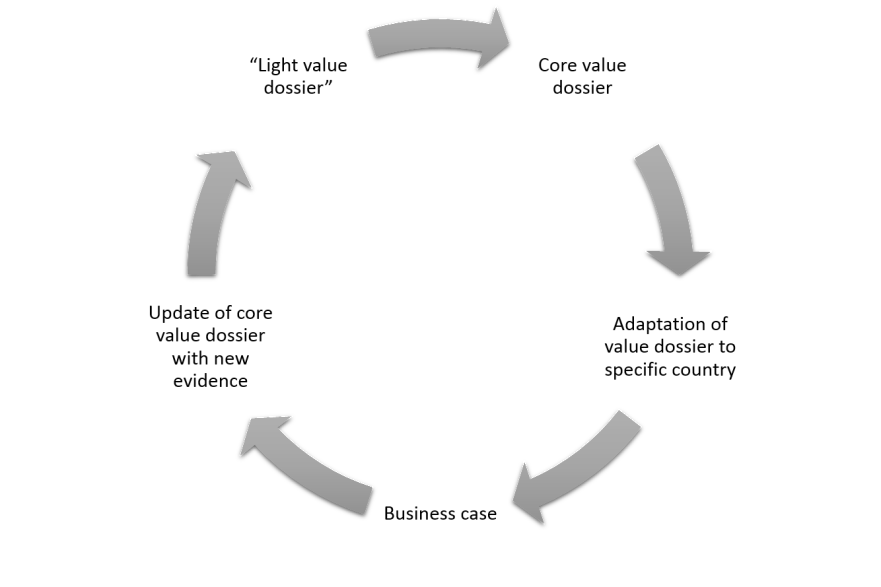
Typically, industry would consider development of value documentation as a process, starting from compiling of “light” dossier, expanding to core value dossier, when more evidence is available and while facing reimbursement/HTA submission, adapting of dossier to different geographies and updating it, when new evidence is available.
Synergus can provide support with development of any format of value documentation.

