Breast Cancer – Part 3
In this blog series we are analysing different aspects of the transferability of disease outcomes, either from RCT to Real-World setting or between different settings/countries. If you missed the prior blog posts in this series, they are available here. 
Here, we continue looking at the issues of transferability and health care delivery with regards to Breast Cancer research.
Regional differences within Sweden
Swedish data shows a substantial difference in the distribution of health care service delivery, by regions. The graph below demonstrates the time difference between the first suspicion of breast cancer and the first visit to a specialist, including the first treatment. Moreover, it presents the time difference between the biopsy date and the first treatment. This data was extracted manually from a Swedish breast cancer quality register. It shows the lowest and highest median days, the range and national median of each abovementioned variables (link).
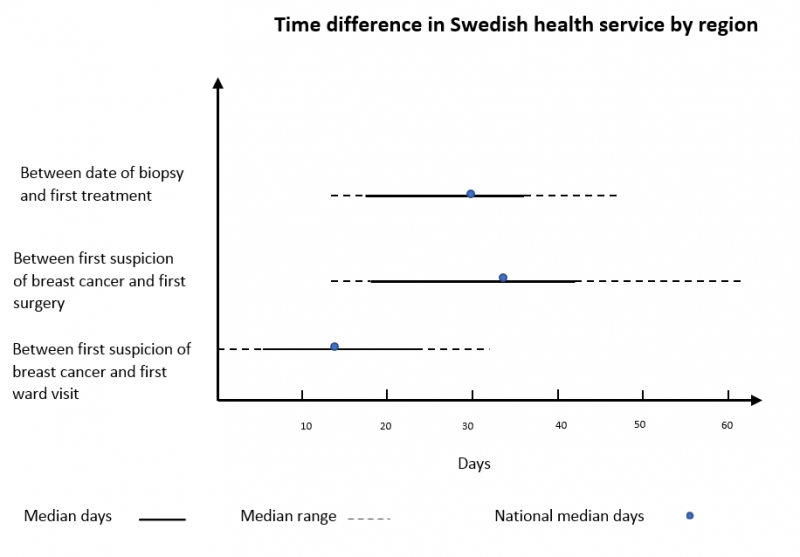
Another Swedish study (1) found that even fairly increased intervals between the morphological diagnosis and first surgery is associated with reduced survival. For instance, a surgical treatment performed 6 weeks after diagnosis, increases the risk of dying due to all the causes which are around 1.3 folds, compared to surgeries performed within 3 weeks.
Studying the drug utilization in Sweden (2) has shown a markable difference in Trastuzumab utilization between health centres in Sweden. It was concluded that among others, the difference could be due to the variety in health services, budget or in the medical providers’ experience and training.
Examples comparing different countries
Using the following graph from the Dreck et al. (3) study, we are trying to demonstrate the variety of treatment strategies and survival outcomes throughout Europe.
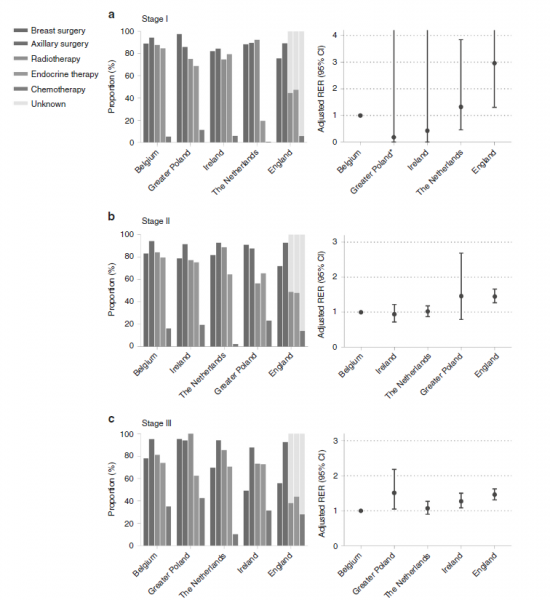
The authors performed the study among elderly patients with breast cancer in 5 European countries, and they concluded that there is a substantial difference between the health strategies applied in Europe, which lead to a variety of survival outcomes. They reported that care service delivery could be related to the type of diseases. For instance, endocrine therapy shows a large variation among patients with stage 1 breast cancer while lower proportions of omitting surgery were associated with higher survival in advanced stages of breast cancer.
A study from the UK (4) investigated the transferability of outcomes from the UK to the Netherland. The study concluded that transferability is possible, however it needs at least 3 steps to be matched with a new situation: 1- Assess transferability, 2- Adapt factors that limit transferability, 3- Estimate country-specific cost-effectiveness.
RCT vs RWE
A Swedish study (5) investigated the external validity of trial data (SENOMIC trial) versus the national breast cancer quality register in Sweden. We think this is a good example of considering the association between the results from a highly controlled environment (RCT) with the results from a real-world dataset. The results of their study are shown below.
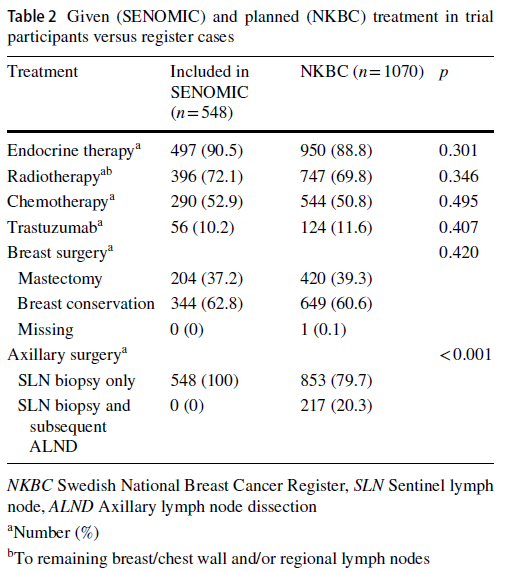
They considered different types of breast cancer treatments in both RCT and national register of breast cancer population and found an acceptable accordance between them. However, they emphasized that there are differences that need to be taken into account when one is implementing the results.
In summary, due to the broad variety in health care delivery throughout Europe and even within a country (Sweden in this case) as well as the results from our ongoing analysis of disease outcomes, it is suggested that the transferability of data outcomes is significantly impacted by health care delivery.
- For updates on our findings, please join the LinkedIn group (Evaluating Transferability of Real World Evidence) where we look forward to your feedback as we share further insights.
- Feel free to also comment on the LinkedIn post.
References:
- Eriksson L, Bergh J, Humphreys K, Wa F. Time from breast cancer diagnosis to therapeutic surgery and breast cancer prognosis: A population‐based cohort study. Cancer Epidemiology. 2018;(5):13.
- Wilking U, Jönsson B, Wilking N, Bergh J. Trastuzumab use in breast cancer patients in the six Health Care Regions in Sweden. Acta Oncologica. 2010 Aug;49(6):844–50.
- Derks MGM. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. :9.
- Essers BAB. Transferability of Model-Based Economic Evaluations: The Case of Trastuzumab for the Adjuvant Treatment of HER2-Positive Early Breast Cancer in the Netherlands. :6.
- Andersson Y, Bergkvist L, Frisell J, de Boniface J. Do clinical trials truly mirror their target population? An external validity analysis of national register versus trial data from the Swedish prospective SENOMIC trial on sentinel node micrometastases in breast cancer. Breast Cancer Res Treat. 2019 Sep;177(2):469–75.

