In a recent post by G-BA, they reinforced to the hospitals are required to submit a 137(h) application for medical devices of class IIb or III, unless G-BA already has clarified that the product is not eligible. It is not up to the hospital (or Medtech company) to determine the eligibility.
53 % of the products who has been assessed for eligibility for 137(h) did not meet the requirements. Seven of these became aware after completing the full 137(h) application and 20 through the consultation process, prior to the submission of the NUB / 137(h).
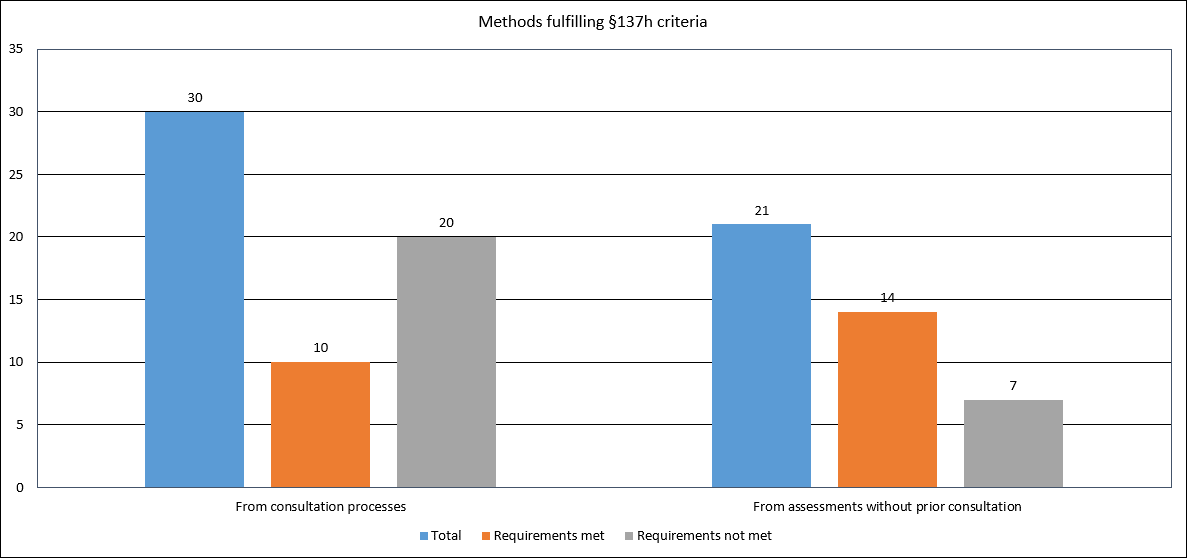
Review result by AiM
For the companies that are eligible for 137(h) the application is a strategic document that requires significant effort to ensure that G-BA understand the technology properly. Considering the description to qualify for eligibility, the safety of the product and finally how to prepare for a potential government sponsored clinical trial.
If you don’t anticipate that you will be eligible for 137(h), this is a rather significant effort which may be avoided. The way to avoid this is to request an assessment by G-BA regarding the eligibility of the technology.
For technologies who may qualify for 137(h), it may be essential to ensure eligibility to establish the government sponsored clinical trial to develop the required evidence for coverage.
The criteria to qualify for 137(h) is:
- First time NUB-application
- Technology based on high-risk medical device (IIb or III) with an especially invasive character.
- New theoretical scientific concept (different than criteria for NUB)
For most companies, the question will be the definition of new scientific concept that creates uncertainty. It can either be a new principle or utility of a previously established method in a new indication. Read more at G-BA.
If you are uncertain about your situation, don’t hesitate to reach out.
- Synergus RWE
- AiM (a sister company in the IGES group focused on market access in Germany)

