Germany introduced a new legislation in 2016 that requires an assessment of the potential benefit of new methods based on high-risk medical devices before they can receive innovation funding (NUB). Results granting NUB can either be proven benefit or potential benefit. The latter requires a coverage with evidence trial partially funded by industry. The third alternative is that the technology is considered to have no potential benefit. In this case, the authorities will start a process to exclude the technology from the hospital setting.
In the first results of this annual process, Holaira’s targeted lung denervation in COPD and Haifu’s ultrasound-steered HIFU in 5 out of 7 indications are considered to have no potential.
The Federal Joint Committee (G-BA) released the results for the first eight § 137h assessments for high-risk technologies based on the information submitted by hospitals and manufacturers. Two technologies show potential for a required treatment alternative while for the other six the G-BA has neither found evidence on benefit nor potential. The G-BA will immediately start internal § 137c consultations on the exclusion of these six methods without potential from the hospital benefit catalogue, these are:
- Ultrasound-steered high intensity focused ultrasound for the treatment of endometriosis of the uterus
- Ultrasound-steered high intensity focused ultrasound for the treatment of malign neoplasm of the pancreas not treatable with surgery
- Ultrasound-steered high intensity focused ultrasound for the treatment of primary malign neoplasm of the bone and cartilage not treatable with surgery
- Ultrasound-steered high intensity focused ultrasound for the treatment of secondary malign neoplasm of the bone and cartilage not treatable with surgery
- Ultrasound-steered high intensity focused ultrasound for the treatment of secondary malign neoplasm of the liver and the intrahepatic bile ducts not treatable with surgery
- Targeted lung denervation through catheter ablation in chronic obstructive pulmonary disease
For the following methods, the G-BA could identify potential benefit and will initiate the § 137e process for a coverage with evidence trial:
- Ultrasound-steered high intensity focused ultrasound in patients with leiomyoma of the uterus
- Ultrasound-steered high intensity focused ultrasound in patients with liver cell carcinoma not treatable with surgery
G-BA reasons potential for TLD in COPD cannot be deduced due to non-transparent and incomplete submission of evidence
Holaira’s submitted evidence is from few studies with small patient populations and short follow-up time (Figure 1). However, the G-BA bases its decision largely on the lack of submitted data or transparency and the resulting inability to deduce benefit or potential. Various patient-relevant endpoints, which were assessed in the studies, were not listed in the submitted dossier. This result underlines the importance of a well-prepared scientific dossier when entering the § 137h process.

Figure 1: Evidence review of Holaira's targeted lung denervation through catheter ablation in COPD
Compared to Holaira, Haifu’s overall published evidence on ultrasound-controlled HIFU ablation in neoplasm and diseased tissue had a larger number of published studies, larger patient cohorts, longer follow-up times, and includes one RCT with 33 patients (Figure 2). Nevertheless, for most indications considered, the evidence was not sufficient or studies were not suitable to assess benefit or potential.
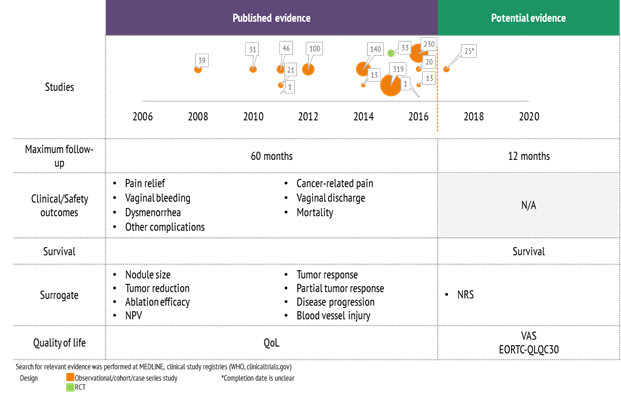
Figure 2: Evidence review of Haifu's ultrasound-controlled HIFU ablation in neoplasm and diseased tissue
Background
Germany’s healthcare system has traditionally been very innovation-friendly with easy access to the market in comparison to other European countries. However, with the introduction of § 137h to the SGB V in 2016, the hospital setting was vastly disrupted. New examination and treatment methods significantly based on high-risk medical devices must now undergo a shortened benefit assessment if a hospital applies for innovation funding (NUB). The G-BA in collaboration with the Institute for Quality and Cost Effectiveness in the Health Care Sector (IQWIG) assesses whether the high-risk method has the potential for a required treatment alternative in the German hospital setting. The assessment can result in three decisions by the G-BA:
- Proven benefit: The method will be included in the whitelist of G-BA’s guideline on methods in hospital treatment
- No proven benefit, but the method shows potential: A § 137e high-quality trial study (RCT) will be initiated, with manufacturers only covering the overhead costs
- No benefit and no potential: A § 137c process will be initiated, eventually excluding the method from the hospital setting by putting it on the blacklist of G-BA’s guideline on methods in hospital treatment

